External Research Studies

Investigator Initiated Studies (IIS)
Investigator Initiated Studies (IIS) are unsolicited independent research studies conducted under the sole responsibility of a non-commercial sponsor — such as an individual, institution, or organization. The sponsor assumes full responsibility for the initiation, management, financing, and operationalization of the study, including direct involvement in activities such as site selection, staff training, and monitoring.
External Collaborative Research (ECR)
External Collaborative Research (ECR) are collaborative research studies conducted under the sole responsibility of a non-commercial sponsor — such as an individual, institution, or organization. The sponsor assumes full responsibility for the initiation, management, financing, and operationalization of the study, including direct involvement in activities such as site selection, staff training, and monitoring. Boehringer Ingelheim may contribute complementary expertise and provide intellectual input in specific areas defined by the ECR Agreement, including:
Study Objectives
Study Design
Protocol Development
Data Ownership
Intellectual Property
Statistical Analysis Plan
Study Report & Publication (Co-Authorship)
The sponsor retains ultimate authority over the conduct of the research, makes all final decisions, and owns the outcomes of all decisions made throughout the study. Additionally, the sponsor is responsible for ensuring compliance with all applicable legal and regulatory requirements. Boehringer Ingelheim is explicitly excluded from any role in the operational execution of the study.
External Research General Information
External Research Studies include externally regulated and externally legally sponsored:
Clinical Trials (CTs): Pre- and post-marketing interventional trials conducted in compliance with current International Conference on Harmonisation Good Clinical Practice (ICH-GCP) guidelines,
Non-interventional studies, including observational and Real-World Evidence (RWE) studies,
Non-Commercial Health Economics and Outcomes Research (HEOR)
External Research Proposal Process
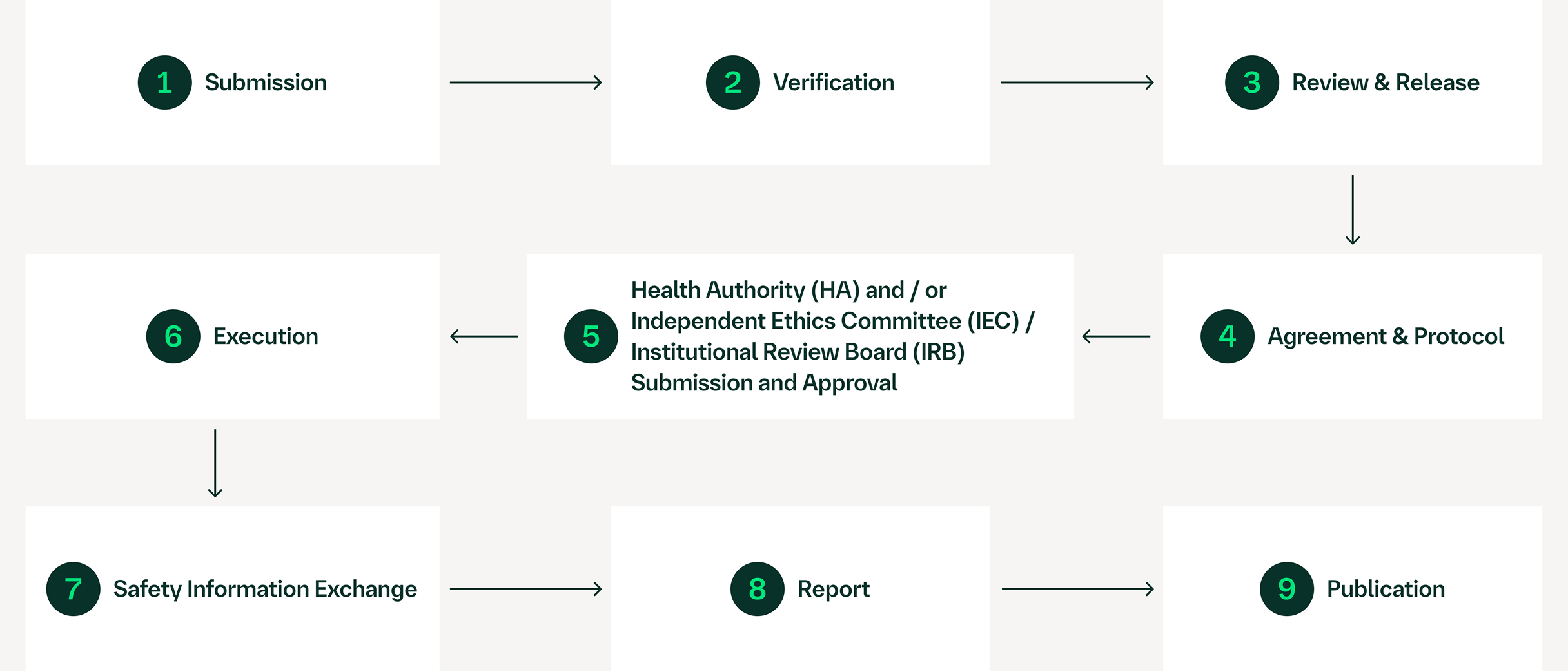
For more information in our areas of focus, visit Human Health Innovation.
For US-specific External Research Information, visit Investigator Initiated Studies | BIPI Medical & Clinical Resources
